Conjugated systems absorb uv light. select the true statement – Conjugated systems, characterized by alternating single and double bonds, possess unique optical properties that enable them to absorb ultraviolet (UV) light. Understanding the relationship between conjugation and UV light absorption is crucial for various applications in chemistry, materials science, and biotechnology.
This comprehensive guide explores the factors influencing UV light absorption, including conjugation length, substituents, and solvent polarity. It also discusses experimental techniques and theoretical models used to study this phenomenon, providing a thorough understanding of the underlying principles.
Conjugated Systems Absorb UV Light: Conjugated Systems Absorb Uv Light. Select The True Statement

Conjugated systems are molecules or ions that contain alternating single and double bonds. This unique structure allows for the delocalization of electrons, resulting in the formation of molecular orbitals that span the entire conjugated system.
UV light absorption occurs when a molecule absorbs a photon of ultraviolet radiation and transitions to an excited electronic state. The energy of the absorbed photon corresponds to the difference in energy between the ground and excited states.
Conjugated systems have a strong tendency to absorb UV light due to their ability to delocalize electrons. The delocalized electrons allow for a more efficient absorption of UV photons, leading to a higher absorption intensity.
Factors Affecting UV Light Absorption
Conjugation Length
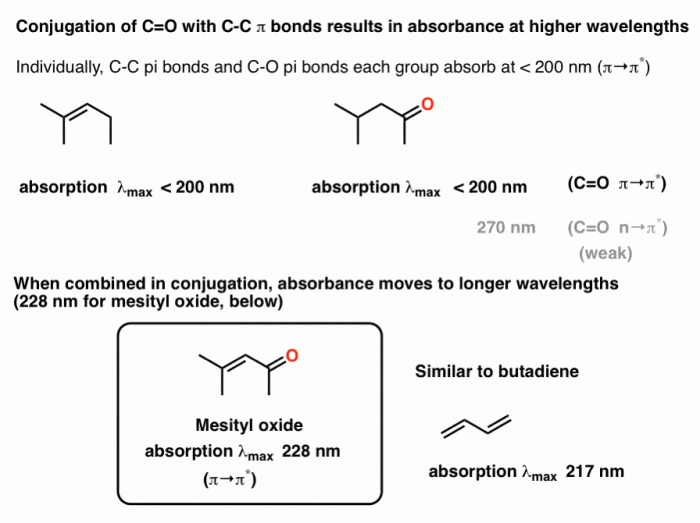
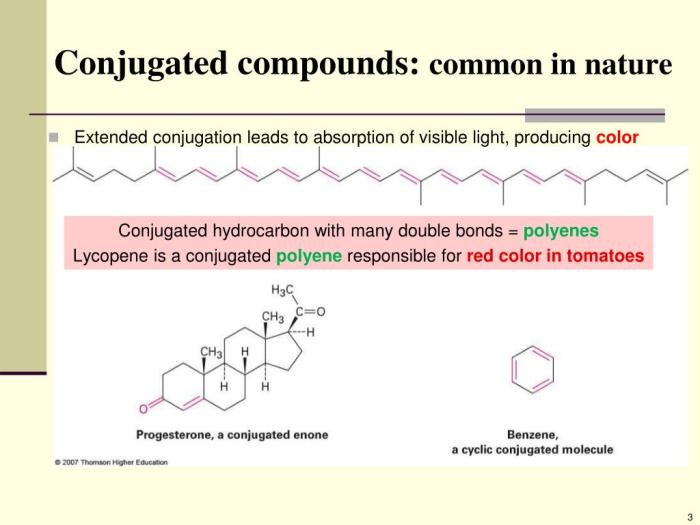
The length of the conjugated system plays a significant role in UV light absorption. As the conjugation length increases, the energy difference between the ground and excited states decreases, resulting in a redshift in the absorption wavelength.
Substituents
The presence of substituents on the conjugated system can affect UV light absorption. Electron-donating substituents, such as alkyl groups, tend to increase the absorption wavelength, while electron-withdrawing substituents, such as carbonyl groups, tend to decrease the absorption wavelength.
Solvent Polarity
The polarity of the solvent can also influence UV light absorption. In polar solvents, the solute-solvent interactions can stabilize the excited state, leading to a redshift in the absorption wavelength. Conversely, in nonpolar solvents, the solute-solvent interactions are weaker, resulting in a blueshift in the absorption wavelength.
Applications of UV Light Absorption by Conjugated Systems, Conjugated systems absorb uv light. select the true statement
Organic Chemistry
UV light absorption is a valuable tool for identifying and characterizing organic compounds. The absorption wavelength and intensity can provide information about the structure and functionality of the molecule.
Materials Science
Conjugated systems are used in a variety of materials science applications, such as organic solar cells, light-emitting diodes (LEDs), and photochromic materials. The ability of conjugated systems to absorb and emit light makes them ideal for these applications.
Biotechnology
Conjugated systems are also used in biotechnology, such as in DNA sequencing and fluorescence microscopy. The ability of conjugated systems to absorb and emit light makes them useful for labeling and imaging biological molecules.
Experimental Techniques for Studying UV Light Absorption
UV-Vis Spectroscopy
UV-Vis spectroscopy is a technique that measures the absorption of light by a sample in the ultraviolet and visible regions of the spectrum. This technique can be used to determine the absorption wavelength and intensity of a conjugated system.
Fluorescence Spectroscopy
Fluorescence spectroscopy is a technique that measures the emission of light by a sample after it has absorbed light. This technique can be used to study the excited state properties of a conjugated system.
Other Techniques
Other techniques that can be used to study UV light absorption by conjugated systems include photoluminescence spectroscopy, Raman spectroscopy, and time-resolved spectroscopy.
Theoretical Models for UV Light Absorption
Molecular Orbital Theory
Molecular orbital theory is a theoretical framework that can be used to explain the electronic structure and properties of conjugated systems. This theory provides a qualitative understanding of the absorption and emission of light by conjugated systems.
Pi-Electron Delocalization
Pi-electron delocalization is a key concept in understanding the UV light absorption of conjugated systems. Pi-electrons are the electrons that are involved in the double bonds of the conjugated system. The delocalization of these electrons allows for the formation of molecular orbitals that span the entire conjugated system.
Relationship Between Conjugation and Energy Levels
The conjugation of a system affects the energy levels of the molecular orbitals. As the conjugation length increases, the energy difference between the ground and excited states decreases. This decrease in energy difference leads to a redshift in the absorption wavelength.
Common Queries
What is the significance of conjugation length in UV light absorption?
Conjugation length plays a crucial role in determining the wavelength of maximum absorption. Longer conjugated systems exhibit absorption at longer wavelengths due to increased delocalization of pi-electrons.
How do substituents affect UV light absorption?
Electron-donating substituents tend to red-shift the absorption maximum, while electron-withdrawing substituents blue-shift it. This is attributed to changes in the energy levels of the molecular orbitals.
What is the role of solvent polarity in UV light absorption?
Solvent polarity can influence the absorption spectrum of conjugated systems. Polar solvents stabilize polar excited states, leading to a red-shift in the absorption maximum.